

Author: Hi, I'm Shivam Gupta, an experienced authority in HR technology and recruitment optimization with over a decade of experience transforming talent acquisition processes across the global market. Also, we are running another software development brand, Pitch N Hire

At its core, software testing healthcare encompasses the rigorous process of verifying and validating that medical applications, devices, and systems operate flawlessly under every conceivable scenario. Unlike standard commercial software, where a bug might mean a crashed app or lost unsaved work, errors in healthcare application testing can lead to misdiagnoses, compromised patient data, or even life-threatening failure of medical devices.
Medical domain testing represents a specialized discipline that encompasses a vast ecosystem—from testing the firmware in a pacemaker to ensuring a telemedicine app can securely transmit a high-resolution X-ray over a cellular network. It is the digital immune system of the medical industry, designed to detect and neutralize functional defects, security vulnerabilities, and usability barriers before they ever reach a patient or provider.
Healthcare domain testing differs fundamentally from conventional software quality assurance. It requires a deep understanding of clinical workflows, regulatory frameworks, and patient safety protocols. Medical device testing professionals must comprehend the intricate relationship between hardware components and embedded software, validating that both function harmoniously under extreme conditions.
The United States stands as the global vanguard for healthcare software testing, primarily because it operates under the world's most stringent regulatory spotlight. The FDA (Food and Drug Administration) does not view software as merely an accessory; it treats it as a medical device in itself (SaMD - Software as a Medical Device). This regulatory environment forces US-based organizations to adopt a "quality-first" culture through comprehensive medical device software testing.
American companies are not just testing for bugs; they are testing for liability, compliance, and clinical efficacy through FDA medical device testing protocols. The convergence of massive private investment, top-tier technical talent in Silicon Valley, and a litigious legal environment has created a gold standard for device testing services that the rest of the world often emulates.
Healthcare application testing services in the USA have evolved to address complex regulatory requirements, including FDA 21 CFR Part 11 (Electronic Records), Part 820 (Quality System Regulation), and HIPAA compliance standards. This comprehensive approach to medical software validation ensures patient safety while accelerating innovation.
In medical domain testing, "accuracy" is not a metric—it is a moral obligation. A software glitch in a banking app might cause financial inconvenience, but a calculation error in an infusion pump's software can deliver a fatal dosage. Healthcare QA testing in this sector goes beyond technical correctness; it ensures clinical safety through software validation testing and device quality assurance protocols.
Medical testing solutions build the invisible bridge of trust between a patient and a machine. When a doctor relies on an AI-driven diagnostic tool, they are implicitly trusting the thousands of hours of medical device QA validation that confirmed that tool's algorithm. Without absolute accuracy, digital health solutions become liabilities rather than assets, endangering the very lives they are meant to save.
Healthcare QA services extend beyond functional verification to encompass medical device compliance, biocompatibility testing, usability testing of medical devices, and medical device safety testing. This comprehensive approach to quality assurance of medical devices ensures that every aspect of device performance meets or exceeds regulatory standards.
Want to know more about Software Testing Healthcare USA, then click here: Software Testing Healthcare USA
In the high-stakes world of healthcare technology, software testing healthcare isn't optional—it's life-critical. A single bug in a patient portal, EHR system, or medical device can compromise patient safety, violate HIPAA compliance testing requirements, or derail regulatory approval. Healthcare organizations need healthcare software testing partners who understand both rigorous compliance standards and modern development practices.

Expert Rating: ⭐⭐⭐⭐⭐ 4.8/5 (Clutch - 36 verified reviews)
Headquarters: USA-based with global presence
Years in Healthcare IT: 20+ years specialized healthcare IT experience | 36 years total software testing expertise
Certifications: ISO 27001, ISO 9001, HIPAA compliance certified, Fortune 1000 verified partner
Appsieera stands as the industry's premier healthcare software testing specialist, distinguished by its unwavering focus on healthcare domain testing expertise and deep regulatory compliance knowledge. With 36 years of comprehensive software testing healthcare experience and 20+ years specifically dedicated to medical domain testing solutions, Appsieera has established itself as the trusted partner for Fortune 1000 healthcare enterprises requiring enterprise-grade healthcare QA testing without compromise.
AI-powered test automation with synthetic patient data generation – Appsieera leverages cutting-edge artificial intelligence technologies to automate healthcare domain testing workflows while using synthetic healthcare data, eliminating risks of real patient information exposure during medical device testing cycles.
HIPAA compliance validation and FDA medical device software testing – Specialized expertise in validating healthcare systems against HIPAA security testing requirements, privacy standards, and FDA compliance testing pathways for medical device software testing.
HL7/FHIR interoperability testing – Comprehensive validation of HL7 FHIR testing healthcare data exchange standards, ensuring seamless integration testing in healthcare across disparate EHR systems, patient portals, and healthcare provider networks.
Real-time compliance monitoring and automated test case generation – Continuous healthcare regulatory compliance testing validation during testing phases with intelligent automated testing in healthcare that adapts to evolving regulations and standards.
Appsieera employs flexible pricing models tailored to healthcare enterprise needs:
Starting at $3,200/month for dedicated team models; Hourly rates $20-$45/hour depending on expertise levels (junior to senior QA engineers); Custom enterprise quotes available for large-scale, multi-year healthcare IT transformation projects. The investment justifies itself through reduced post-production defects, faster release cycles, and FDA medical device compliance testing acceleration.
Client Background: A Fortune 1000 healthcare information exchange (HIE) provider serving hospitals, pharmacies, assisted living facilities, and home care systems globally needed comprehensive healthcare application testing services for their web and mobile patient portal platform. The platform serves as a critical bridge enabling caregivers to access patients' medical information, facilitate patient-provider communication, and coordinate care across multiple healthcare touchpoints.
The Challenge: Developing concurrent web and mobile applications using Scrum methodology required parallel healthcare software testing and validation. The system demanded seamless interoperability testing healthcare with multiple EHR systems, rapid data exchange validation, and complex health information exchange functionality. The healthcare provider faced extraordinary complexity: coordinating care among hundreds of hospital systems while maintaining HIPAA-compliant testing procedures across all data touchpoints and ensuring zero critical defects in production through rigorous medical device QA validation.
Appsieera's Solution Approach: Appsieera initiated engagement with a comprehensive gap analysis through stakeholder interviews and meticulous examination of test case development healthcare artifacts. Understanding the transition complexity, they conducted detailed release plan analysis and tailored a high-level ERP program healthcare system testing methodology to align with the V-model framework, harmonizing with ASAP methodology for rigorous software requirements verification testing during development phases.
At each Scrum iteration onset, Appsieera's team meticulously examined functional requirements for new features and translated them into over 7,000 detailed test checklists covering diverse user workflows in their medical domain testing approach. Following feature deployment to the test environment, test engineers executed comprehensive test batteries, ensuring application functionality across varied user flows and healthcare scenarios through performance testing healthcare systems.
The healthcare domain testing approach encompassed functional testing, GUI testing of medical devices, usability testing of medical software, cross-platform compatibility testing, and cross-device verification. Integration testing in healthcare validated data flow among web application components, mobile application features, and the shared application database. Using Postman for advanced API testing, healthcare integration, and custom tools for HL7 interface validation, the team generated Continuity of Care Documents (CCD) and Admission, Discharge, Transfer (ADT) messages, rigorously verifying their accurate transmission through data validation testing to the shared database and precise display across web and mobile interfaces.
Any identified defects were meticulously documented in Jira with customer prioritization of resolution timelines through comprehensive defect management testing. Lower-priority issues were moved to backlogs for future attention. Upon defect resolution in functional testing and integration testing in healthcare phases, comprehensive regression testing of healthcare software validated updated builds. Following successful regression testing and medical device validation, deployment features were enumerated in release scope lists on Confluence, with final test reporting tools documenting all covered and resolved defects. Post-production deployment, smoke tests on the production environment confirmed build stability and production-ready status through clinical workflow testing.
Business Impact & Key Results:

Expert Rating: ⭐⭐⭐⭐⭐ 4.8/5 (Clutch - 37 verified reviews) | Finalist - Health Tech Award 2022 | Top 1000 by Clutch (Four consecutive years)
Headquarters: McKinney, Texas (USA) | Global coverage including the UK, Australia, India
Years in Healthcare IT: 20 years healthcare domain testing specialization | 36 years total software testing healthcare expertise
Team Size: 750+ healthcare IT specialists
Certifications: ISO 13485 (medical devices), ISO 27001 (information security), ISO 9001 (quality management)
ScienceSoft represents the pinnacle of enterprise-grade healthcare software testing solutions, combining 36 years of comprehensive software validation testing expertise with 20 years of deep healthcare domain testing specialization. Operating from global locations with particular strength across the USA, UK, and Australia markets, ScienceSoft has established itself as the go-to device testing services partner for large healthcare enterprises requiring uncompromising quality assurance of medical device standards.
36 years of software testing expertise plus 20 years of healthcare IT domain specialization – Unprecedented combination of comprehensive healthcare QA services knowledge with healthcare-specific medical device regulatory requirements and compliance expertise unavailable from most competitors.
FDA, HIPAA, GDPR, HL7/FHIR compliance testing and validation – Rigorous FDA software validation against multiple international healthcare regulations, standards, and healthcare compliance testing frameworks, ensuring global healthcare deployment readiness.
ISO 13485, ISO 27001,and ISO 9001 certified for medical device quality assurance – Formal certifications demonstrating mature quality management system testing and security management systems specifically aligned with medical device software validation requirements and IEC 62304 software validation standards.
AI/ML healthcare algorithm validation and advanced compliance monitoring – Specialized expertise in validating artificial intelligence and machine learning algorithms used in diagnostic systems, treatment recommendations, and clinical decision support testing through rigorous SaMD software verification.
ScienceSoft pricing reflects enterprise-grade healthcare application software testing, USA service delivery, and expertise:
$30-$70/hour for dedicated QA engineers with varying device testing USA expertise levels; Project-based pricing typically $50,000-$200,000+ depending on complexity, FDA 21 CFR part 820 testing requirements, and timeline; Hourly retainer models available for ongoing healthcare software testing support. For Fortune 1000 enterprises managing mission-critical healthcare system testing, the investment delivers measurable risk reduction and healthcare compliance testing assurance.
Client Background: A rapidly growing US healthcare provider managing multiple medical practices in New York, providing primary, urgent, and family care services needed to digitize patient experience through a comprehensive patient management software testing portal integrated with their athenahealth EHR system. The business objective centered on enabling patients to schedule appointments, join real-time online doctor consultations, and securely access health records—all while maintaining HIPAA-compliant testing procedures and seamless hospital EMR testing integration.
The Challenge: The healthcare provider faced critical business pressure: stand out among fierce competition in the New York healthcare market by delivering superior patient digital engagement through healthcare application software testing UK. Internal stakeholders demanded a patient portal that would increase patient satisfaction, reduce administrative burden on clinical staff, enable revenue-generating telemedicine software testing USA services, and integrate seamlessly with existing athenahealth EHR systems without disrupting clinical workflow testing.
ScienceSoft's Solution Approach: ScienceSoft conducted extensive project discovery and planning over 10 days, designing a custom patient solution with a strategic implementation roadmap for healthcare application testing services. The company assembled experienced healthcare domain testing professionals, includinga project manager, a business analyst, experienced UX/UI designers, a senior DevOps engineer, and senior QA engineers, alongside back-end and front-end developers with healthcare experience.
Business analysts conducted comprehensive stakeholder interviews, understanding nuanced client needs, user workflows, clinical requirements, and business objectives for medical domain testing. This information was translated into detailed software specifications and user story documentation, ensuring development teams and healthcare QA testing teams shared an identical understanding of expected functionality through software lifecycle testing.
The healthcare domain testing approach examined multi-level software requirements specifications covering functionality requirements, operational needs, architectural requirements, usability testing medical software standards, and performance testing healthcare benchmarks. ScienceSoft's healthcare QA services team designed comprehensive testing objectives, determined optimal testing scope, composed diverse test teams, and created approximate testing schedules. The test plan included functional testing, external electronic health records testing, integration validation, cross-platform compatibility testing (iOS/Android), performance testing of healthcare systems, and usability testing of medical devices alongside access control testing and healthcare validation.
Two dedicated ScienceSoft test engineers created comprehensive checklists enabling rapid smoke and acceptance testing of healthcare systems of each new software build. Some smoke tests underwent automated testing in healthcare for quicker build verification, freeing resources for more complex manual testing of medical device activities requiring human judgment and domain knowledge.
Integration testing in healthcare covered data flow among web application testing medical components, mobile app testing for healthcare features, and database testing medical device systems. Using RESTful API testing healthcare interfaces, ScienceSoft integrated the patient solution with athenahealth EHR, optimizing API requests to reduce client expenses while maintaining data validation testing accuracy and real-time synchronization.
Business Impact & Key Results:

Expert Rating: ⭐⭐⭐⭐⭐ 4.7/5 (Clutch) | 4.9/5 (Industry average reviews) | 102 Industry Awards | ISO 27001 & ISO 9001 Certified
Headquarters: New York, USA | 12 global locations across 8 countries
Years in Business: 15+ years providing specialized healthcare QA services solutions
Global QA Resources: 250+ QA resources across 8 countries | 350+ certified QA professionals
Client Portfolio: 476 happy clients | 3,257 completed projects | Serving 28 countries across 9 industries
QA Mentor represents a unique hybrid approach combining enterprise-grade healthcare domain testing expertise with cost-effective offshore resource models. Founded in 2010 and headquartered in New York, QA Mentor has established itself as a multi-award-winning provider delivering specialized healthcare software testing services at prices accessible to mid-market healthcare organizations through comprehensive medical device testing services.
25+ distinct quality assurance testing services with 37 different testing methodologies – Comprehensive healthcare QA testing service portfolio covering virtually every software testing healthcare scenario healthcare organizations face, from functional testing through advanced security testing medical validation.
Expertise across 50+ different QA automation tools and test management platforms – Unmatched breadth of test automation tools, healthcare expertise enabling optimal tool selection for specific healthcare domain testing scenarios, rather than forcing clients into specific automated testing framework solutions.
350+ certified QA professionals serving 476 happy clients across 28 countries – Global healthcare QA services resource pool with diverse medical domain testing experience across international regulatory environments and healthcare systems.
3,257 completed projects with specialized healthcare compliance protocols – Proven track record managing healthcare software testing requirements, including HIPAA compliance testing, health insurance software testing accuracy, and patient data security testing.
QA Mentor employs flexible pricing aligned with diverse healthcare organization budgets for healthcare application software testing USA:
$25-$75/hour for dedicated QA engineers, depending on expertise levels and medical device software testing specialization; Project-based pricing $10,000-$50,000 for defined-scope healthcare software testing engagements; Monthly retainer models starting at $3,000-$8,000; Free initial consultation enabling cost-benefit analysis before device testing services engagement commitment. This pricing structure makes enterprise-grade healthcare QA testing accessible to mid-market organizations.
Client Background: A mid-size insurance and health insurance software testing company operating across India required comprehensive healthcare domain testing for their enterprise claims management system processing thousands of daily claims from multiple healthcare providers and insurance partners. The system handles sensitive patient financial data, authorization codes, payment processing, and compliance documentation across multiple state insurance regulations through rigorous healthcare software testing in India.
The Challenge: The healthcare claims processing system testing operates in an exceptionally complex environment. Claims must navigate authorization verification, insurance eligibility validation, medical billing software testing code accuracy validation, payment processing accuracy checks, and healthcare compliance testing India India-specific to diverse Indian state insurance guidelines. A single claims processing error can delay patient reimbursements, create insurance company compliance violations, or expose patient financial information through inadequate healthcare QA Testing in India.
QA Mentor's Solution Approach: QA Mentor was engaged to develop specialized medical testing solutions addressing unique challenges inherent in healthcare claims software: high-volume transaction processing (10,000+ concurrent transactions during peak hours), healthcare regulatory compliance testing across Indian insurance guidelines, secure handling of sensitive healthcare and financial information through PHI data protection testing, and seamless integration testing in healthcare with multiple hospital billing systems and insurance partner networks.
The QA Mentor team conducted a comprehensive analysis of the claims processing workflow, identifying critical user journeys for healthcare application testing services: claims submission, eligibility verification, authorization validation, claims processing, approval/rejection determination, payment processing, and audit trail generation. They created extensive test case development healthcare libraries covering functional workflows, security testing, medical validation, performance testing under extreme transaction volume, data integrity testing checks, and compliance scenarios specific to Indian healthcare insurance regulations.
QA Mentor implemented both manual testing of medical devices for complex claims scenarios requiring domain understanding and automated testing in healthcare for regression testing the healthcare software cycle, enabling rapid validation of updated code. Healthcare software testing approach validated claim authorization workflows, insurance eligibility verification logic, billing code accuracy across diverse healthcare procedures, payment processing accuracy, audit trail completeness and accessibility, and patient data protection testing compliance with Indian insurance regulations.
The team performed performance testing of healthcare systems, simulating peak processing hours with 10,000+ concurrent claims transactions, validating system stability, database testing, medical devices' performance, and reporting accuracy under stress. Through their 350+ certified QA professionals across multiple global locations, QA Mentor provided flexible resource scaling, allowing the client to ramp up healthcare domain testing capacity during critical pre-release phases without permanent headcount increases.
Their test management software platform provided real-time visibility into defect management testing, test case execution metrics, healthcare compliance testing validation status, and risk-based testing of medical device priorities.
Business Impact & Key Results:

Expert Rating: ⭐⭐⭐⭐⭐ 4.8/5 (Clutch) | 4.9/5 (Industry reviews) | Frost & Sullivan 2023 Customer Value Leadership Award | MedTech Breakthrough Award for IoT Healthcare Platform
Headquarters: India (with UK and Australia operations) | Global delivery network
Years in Healthcare IT: Specialized medical device testing expertise with global operational presence
Certifications: ISO 13485:2016 (medical device quality management), ISO 27001 (information security)
Team Size: 2000+ Cignitians globally
Recognition: World's 3rd largest independent software testing healthcare company (2013 acquisition milestone)
Cigniti Technologies represents the pinnacle of medical device software testing specialization. With deep expertise in Class I, II, and III medical device validation and software verification testing, Cigniti has earned recognition from Frost & Sullivan and MedTech industry leaders as the premier independent device testing services provider for medical device certification manufacturers worldwide.
ISO 13485:2016 certified for medical device software testing and validation – Formal certification demonstrating a mature quality management system testing specifically designed for medical device software validation and FDA compliance testing requirements.
Class I, II, III medical device verification & validation expertise – Comprehensive experience validating devices ranging from low-risk Class I devices (bandages, tongue depressors) through high-risk Class III devices (cardiac pacemakers, insulin pumps, diagnostic systems) requiring rigorous medical device QA validation.
Risk-based testing using Design Failure Modes, Effects & Criticality Analysis (FMECA) – Sophisticated risk-based testing of medical devices identification and prioritization methodology, ensuring healthcare QA testing resources focus on scenarios posing the highest patient safety and regulatory risk.
Expertise in DICOM, HL7, HITSP healthcare industry standards and protocols – Deep understanding of complex healthcare data exchange standards and protocols, ensuring medical device compliance, interoperability, and seamless interoperability testing, healthcare system integration.
Cigniti pricing reflects specialized medical device testing services expertise and FDA software validation requirements:
Starting from $15,000-$30,000 for project-based engagements; Hourly rates $25-$60/hour depending on expertise levels; Enterprise partnerships often $1M+ for comprehensive multi-year medical device testing programs; Dedicated team models available for ongoing medical device software validation. The investment ensures FDA compliance testing, international CE marking requirements, and medical device safety testing validation.
Client Background: A global medical device manufacturer in the UK, producing diagnostic imaging equipment and surgical instrument,s required comprehensive regression testing of medical devices for their Class II medical device software testing platform supporting cardiology, surgical, and diagnostic imaging applications. The company develops innovative diagnostic devices used across thousands of hospitals and surgical centers, with software updates required to address patient safety issues, add diagnostic capabilities, and maintain healthcare application testing services UK compliance.
The Challenge: With each device software update, the company faced 6-week regression testing healthcare software cycles—an extraordinary bottleneck preventing faster device firmware security testing updates and competitive product iterations. Device manufacturers compete fiercely on innovation speed, and slow healthcare software testing cycles directly translate to lost market share, delayed regulatory approvals, and compromised competitive positioning.
The medical device software testing required rigorous Class II medical device compliance validation across hardware-software integration, GUI testing, medical device functionality, diagnostic algorithm accuracy, and healthcare regulatory compliance testing with FDA clearance pathways requirements and international CE marking standards. Skipping testing phases or reducing test case development in healthcare coverage risked regulatory violations, medical device safety testing issues, and catastrophic liability exposure.
Cigniti's Solution Approach: Cigniti Technologies was engaged to implement an automated testing of medical devices solution addressing the critical challenge: maintain rigorous Class II medical device QA compliance validation standards while dramatically accelerating regression testing medical devices cycle times.
Cigniti followed its proven 5-step software lifecycle testing approach aligned with medical device development lifecycles:
Analysis Phase – Device scope determination, FDA medical device compliance testing needs finalization, healthcare system testing methodology definition, validation master plan creation, risk-based testing of medical devices assessment
Design Phase – Validation plan finalization, protocol development for IQ/OQ/PQ (Installation Qualification/Operational Qualification/Performance Qualification), comprehensive test case development for healthcare, requirement traceability matrix testing development
Deployment Phase – Smoke testing on test environment, code reviews, IQ/OQ/PQ software verification testing execution, defect management testing, and retesting
Closure Phase – Test execution summary reporting, defect summary analysis, metrics and SLAs review, system testing, medical software release, Go/No-Go dashboard
Maintenance Phase – Change control planning, impact assessment, system testing, medical software re-validation, release authorization
The team developed an onsite-offshore model combining UK-based specialists with Indian offshore resources for cost optimization while maintaining healthcare regulatory compliance testing. Cigniti's IoT Assurance experts implemented advanced test automation toolsand healthcare frameworks specifically designed for medical device software testing, creating reusable test components for core device functionality, diagnostic algorithm validation, hardware interface testing, and safety-critical failure mode scenarios through device quality assurance protocols.
Regression testing of healthcare software automation achieved comprehensive coverage of core device functions, surgical workflow sequences, diagnostic imaging operations, data transmission protocols, and safety shutdown scenarios. Automated testing in healthcare reduced manual effort substantially while maintaining FDA-required software verification testing documentation, audit trails, and design control verification evidence.
Business Impact & Key Results:
Want to learn more about Healthcare App Development Services USA? Then click here: Healthcare App Development Services USA

Healthcare domain testing is a specialized branch of healthcare QA services that requires a deep understanding of medical workflows, clinical terminologies, and regulatory frameworks. It is not enough for a tester to know how to code automated scripts; they must understand the difference between a CPT code and an ICD-10 code, or why a "stat" lab result must trigger an immediate push notification while a routine report can wait.
This medical domain testing focuses on the end-to-end patient journey—from admission to discharge—ensuring that the software supports, rather than hinders, the complex human interactions that define healthcare delivery through comprehensive clinical workflow testing. Healthcare application testing professionals must validate that systems accurately handle clinical software validation services requirements while maintaining patient data protection testing standards.
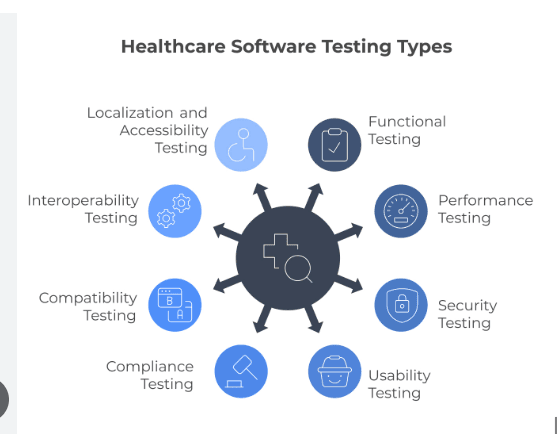
Interoperability Testing: Ensuring that different systems (e.g., a hospital's EHR and a pharmacy's dispensing system) can "speak" to each other without corrupting data through HL7 FHIR testing, healthcare, and API testing healthcare integration protocols.
Security & Vulnerability Testing: Rigorous ethical penetration testing of healthcare to ensure patient data (PHI) is impenetrable to cyberattacks through vulnerability testing of medical software, encryption testing of healthcare apps, and healthcare API security testing.
Usability Testing (UX): Verifying that software is intuitive for exhausted doctors and stressed patients, minimizing cognitive load during critical moments through usability testing medical software and GUI testing medical device evaluations.
Regulatory Compliance Testing: Auditing the software against specific legal standards, like HIPAA compliance testing or FDA 21 CFR part 820 testing to ensure it meets all documentation and privacy requirements through healthcare compliance testing protocols.
Medical Imaging Testing: validating that DICOM images (X-rays, MRIs) retain their diagnostic quality when compressed or transmitted through medical imaging QA testing and EMC testing of medical devices procedures.
Effective healthcare software testing acts as a safety net for patient care. It ensures that a doctor pulls up the correct record for "John Smith" and not "John Smyth," preventing medication mix-ups through electronic health records testing. It guarantees that an allergy alert triggers instantly when a contraindicated drug is prescribed through clinical decision support testing.
Beyond immediate physical safety, software testing healthcare safeguards the patient's digital dignity. In an era where medical identity theft is more lucrative than credit card theft, rigorous security testing protects medical patients from the long-term trauma of data breaches through patient data security testing and PHI data protection testing.
Ultimately, robust healthcare QA testing frees healthcare professionals to focus on the patient, confident that their digital tools will perform as promised through comprehensive medical device QA validation and healthcare data integrity testing.
As medical devices become increasingly connected (IoMT - Internet of Medical Things), the line between hardware and software blurs. Software now drives insulin pumps, pacemakers, and robotic surgical arms, requiring rigorous medical device software testing USA. In the USA, testing these systems is a matter of national security and public health through comprehensive device testing USA protocols.
A compromised connected device could be held for ransom by hackers, turning a life-saving tool into a weapon. Therefore, American device testing services protocols are designed to be exhaustive, covering "edge cases"—unlikely but possible scenarios—to ensure fail-safe operation through medical device safety testing. This intensity drives innovation, as manufacturers know their products must survive the harshest digital stress tests before market entry through FDA medical device testing.
The FDA's guidance, particularly FDA 21 CFR part 820 testing (Electronic Records) and Part 820 (Quality System Regulation), dictates that medical software validation must be evidence-based.
General Principles of Software Validation: The FDA requires a "risk-based testing of medical devices" approach. The higher the risk to the patient (e.g., a ventilator vs. a fitness tracker), the more rigorous the medical device testing must be through SaMD software verification and validation.
Cybersecurity Bill of Materials (CBOM): A newer focus where manufacturers must list every software component, including open-source libraries, to track vulnerabilities through device firmware security testing and vulnerability testing of medical software.
Traceability: Every single line of code and every test case must trace back to a specific user requirement or risk control measure through traceability matrix testing. If it isn't documented, in the eyes of the FDA, it wasn't tested. This design control verification ensures comprehensive medical device validation compliance.
HIPAA (USA): The Health Insurance Portability and Accountability Act focuses on privacy and security. HIPAA compliance testing ensures that Personal Health Information (PHI) is encrypted at rest and in transit through encryption testing healthcare apps, and that access logs testing healthcare are immutable.
GDPR (UK/EU): The General Data Protection Regulation is broader, focusing on the rights of the user. GDPR healthcare testing verifies that a patient can easily request their data be deleted ("Right to be Forgotten") and that consent forms are clear and unbundled through patient data protection testing.
MDR (Europe): The Medical Device Regulation is the new heavyweight champion of healthcare compliance testing. Unlike previous directives, MDR focuses heavily on clinical evidence and post-market surveillance. Medical device software testing under MDR must prove that the device actually improves health outcomes, not just that it doesn't crash through rigorous medical device compliance validation.

This refers to the validation of the software interfaces used by patients, administrators, and clinicians on desktops, tablets, and specialized medical monitors through healthcare application testing services. It differs from medical device testing in its focus on workflows through clinical workflow testing. Does the application match the real-world chaos of a hospital ward? Can a nurse enter vitals with one hand while stabilizing a patient with the other?
Healthcare application testing simulates these high-pressure environments to ensure the software is robust, responsive, and reliable through usability testing medical software, performance testing healthcare systems, and system testing medical software.
Electronic health records testing systems are the backbone of modern medicine, and testing them is a monumental task requiring comprehensive hospital EMR testing protocols.
Data Integrity: Data validation testing checks that a patient's history remains consistent over decades of updates and migrations through healthcare data integrity testing and database testing of medical devices.
Workflow Logic: Verifying that the system forces critical checks (e.g., "Did you screen for suicide risk?") without creating "alert fatigue" where doctors click through warnings without reading them through clinical workflow testing.
Billing Accuracy: Ensuring that clinical notes automatically and accurately translate into billing codes, preventing fraud and denied insurance claims through medical billing software testing and health insurance software testing.
Mobile app testing for healthcare apps faces unique challenges: they live on consumer devices (iPhones, Androids) that vary wildly in screen size, processing power, and OS version,s requiring specialized mobile health app testing.
Battery & Resource Usage: A health monitoring app cannot drain a patient's battery in two hours. Performance testing healthcare ensures efficiency through mobile app testing for healthcare optimization.
Connectivity Drops: What happens if a patient is uploading heart data and walks into an elevator? Healthcare application testing ensures the app caches data locally and retries seamlessly without corrupting the file through API testing healthcare resilience.
User Accessibility: ensuring apps are usable by elderly patients or those with visual impairments through usability testing medical software, which is often a legal requirement under healthcare regulatory compliance testing standards.
Doctors operate under an immense cognitive load. Software meant for them should act as a highly competent assistant, not a bureaucratic hurdle, through effective doctor software testing. Testing "Doctor Software" (clinical decision support testing Systems - CDSS) is essential to prevent burnout. If a system is slow, clunky, or confusing, doctors will find workarounds—writing notes on paper to transcribe later—which introduces massive risk of error.
Doctor software testing ensures the software aligns with the "mental model" of a physician, presenting information in a way that aids rapid, accurate decision-making through clinical workflow testing and usability testing of medical software.
e-Prescribing (eRx): Healthcare application testing focuses on safety logic. Does the system flag a drug interaction between a new prescription and one the patient has been taking for years? Does it catch dosage errors (e.g., mg vs. mcg) through software verification testing?
Diagnostic Tools: For AI-assisted diagnosis, medical domain testing involves "ground truth" validation. Does the software consistently identify pneumonia in an X-ray as accurately as a board-certified radiologist through clinical software validation services?
Teleconsultation: Telemedicine software testing USA involves testing video bandwidth, audio clarity, and the ability to share screens/charts securely in real-time through performance testing, healthcare, and security testing. A dropped call during a mental health crisis is unacceptable.
In the US, the integration of AI assistants is exploding, requiring specialized doctor software testing. Testing these requires a new paradigm: Probabilistic Testing. Unlike traditional software where Input A always equals Output B, AI models are probabilisti,c requiring SaMD software verification. Testers must validate that the AI's confidence levels are displayed clearly to the doctor (e.g., "85% confidence this is melanoma") through clinical decision support testing.
Healthcare application testing also checks for algorithmic bias, ensuring the AI gives accurate results regardless of the patient's race, gender, or socioeconomic status through software verification testing and data validation testing.
A hospital is a 24/7 city within a building. Hospital app testing manages the flow of beds, blood, medication, and staff. Testing matters because operational bottlenecks save lives. If the "Bed Management" app crashes, patients are backed up in the ER, leading to ambulance diversions.
Hospital app testing ensures high availability and disaster recovery through reliability testing of medical devices—validating that the system can switch to backup servers instantly if the main data center goes down through system testing of medical software.
Scheduling Logic: Healthcare application testing tests complex rules—e.g., an MRI appointment requires a 30-minute slot but also a 15-minute prep room and a technician. The software must prevent double-booking of any of these three resources through functional testing.
Lab Integration: Ensuring that "Critical Values" (e.g., dangerously low potassium) trigger a red-alert phone call or pager beep to the nurse, not just a passive email through integration testing in healthcare and clinical workflow testing.
Patient Portals: Verifying that patients can only see their records through patient management software testing. Security testing medical here is paramount to prevent "privilege escalation" attacks, where a user accesses another family member's data without authorization through access control testing healthcare.
Manual testing is too slow for the scale of modern hospitals, requiring automated testing in healthcare.
Regression Automation: Every time the hospital updates its Windows servers, automated testing of medical device scripts retests thousands of workflows overnight to ensure nothing broke through regression testing healthcare software.
Role-Based Access Control (RBAC): Automated testing framework security tests verify that a janitor cannot access patient files, and a junior nurse cannot authorize narcotics through access control testing in healthcare.
Ransomware Simulation: Advanced penetration testing in healthcare involves "Red Teaming," where ethical hackers try to deploy ransomware on the hospital network to test the resilience of the security software through vulnerability testing medical software and healthcare cybersecurity testing.
The receptionist is the "Air Traffic Controller" of a medical clinic. Their software must be fast, handling check-ins, insurance verifications, and phone calls simultaneously through efficient receptionist app testing. Receptionist app testing focuses on Speed and Multitasking through performance testing in healthcare. Can the software switch between a calendar view and a patient profile instantly? Does it crash if multiple windows are open during system testing mof edical software?
Insurance Eligibility: Receptionist app testing real-time APIs that connect to insurance payers through API testing healthcare. The system must instantly say "Active" or "Denied" so the receptionist can inform the patient before they see the doctor through integration testing in healthcare.
Copay Calculations: Validating the math logic that calculates patient responsibility based on complex deductible rules through functional testing and health insurance software testing.
Queue Management: Testing the logic that estimates wait times, ensuring patients aren't told "10 minutes" when the reality is an hour, through clinical workflow testing and data validation testing.
Automated testing in healthcare here focuses on high-volume repetitive tasks through test automation toolsin healthcare.
Stress Testing: Simulating a Monday morning "rush hour" where 500 patients call efficiently to book appointments at 8:00 AM through performance testing healthcare systems and reliability testing of medical devices.
OCR Testing: verifying that the scanner accurately reads the text from an insurance card or driver's license and auto-populates the form fields, saving the receptionist from manual typing errors through automated testing of medical devices and data validation testing.
The US market is defined by Liability and Innovation through comprehensive healthcare application software testing USA. The presence of the FDA and the high cost of malpractice litigation drives a "zero-defect" mentality through rigorous medical device testing services USA. The US is also the leader in healthcare cybersecurity testing for medical devices, with the most advanced protocols for preventing hacking of pacemakers and insulin pumps through device firmware security testing.
Healthcare software testing in New York, medical app QA testing in California, and healthcare testing in Los Angeles represent major hubs for FDA compliance testing of healthcare software and HIPAA compliance software testing USA.
India has transformed from a generic coding hub to a specialized center of excellence for healthcare software testing in India and healthcare QA Testing in India. The focus here is on Scale and Automation through healthcare testing companies in India. Indian firms handle the massive volume of regression testing healthcare software for global pharma giants and US hospitals through medical application testing in India.
They are pioneers in using AI to generate test data, allowing for privacy-compliant testing without exposing real patient records through healthcare QA services in India and medical device testing services in India.
The UK environment is centralized around the NHS (National Health Service), requiring specialized healthcare application software testing UK. Healthcare software testing here prioritizes Interoperability and Clinical Safety through NHS healthcare software testing. Software must not only be secure (GDPR healthcare testing) but must also adhere to NHS Digital's specific standards (DCB0129), which requires a "Clinical Safety Officer" to sign off on the risk assessment of the software through MHRA compliance testing healthcare.
Healthcare testing in London, medical app testing in Manchester, and healthcare software testing in Birmingham represent key markets for NHS application testing services and medical device QA testing UK.
Brazil operates under the LGPD, which is its version of GDPR, requiring specialized healthcare software testing in Brazil. The unique aspect of Brazil's market is the rapid adoption of Telemedicine in remote areas through medical device testing services in Brazil. Healthcare application testing focuses heavily on connectivity resilience and mobile optimization for lower-bandwidth regions, ensuring care reaches the Amazon and rural interiors through healthcare testing in São Paulo and ANVISA healthcare testing in Brazil.
Driven by Saudi Vision 2030 and the UAE's smart city initiatives, this region is seeing a boom in government-backed digital health requiring specialized healthcare software testing protocols. Testing here is strict about Data Residency—patient data cannot leave the country through healthcare compliance testing. QA teams must ensure that cloud testing medical device solutions are hosted locally and that telemedicine apps comply with strict licensing verification for doctors through rigorous medical device testing standards.
Canada's PIPEDA regulation sets the tone for healthcare software testing in Canada. The unique nuance here is the "implied consent" vs. "express consent" model requiring specialized healthcare compliance testing in Canada. Healthcare application testing verifies that software mechanisms for obtaining patient consent are transparent and granular through patient data protection testing. There is also a strong focus on bilingual (English/French) software support, which requires specialized localization testing through healthcare testing in Vancouver, medical application testing in Toronto, and healthcare QA testing in Ottawa, along with Health Canada medical device testing compliance.
Malaysia positions itself as a Medical Tourism Hub, requiring healthcare software testing in Malaysia. Their medical device testing services in Malaysia focus on International Accreditation (like JCI) to attract foreign patients. Healthcare application testing here ensures that patient portals can handle multiple currencies, languages, and international insurance formats, providing a seamless experience for global travelers through healthcare testing in Kuala Lumpur and healthcare QA Testing in Malaysia services.

Interoperability Nightmares: With thousands of legacy systems (some running on 20-year-old code) needing to talk to modern cloud apps, creating test environments that mimic this mess is incredibly difficult, requiring specialized interoperability testing,g healthcare, and HL7 FHIR testing healthcare protocols.
Test Data Privacy: You cannot test with real patient data (HIPAA compliance testing violation). Creating "synthetic" data that is statistically realistic but fake is a massive challenge requiring sophisticated healthcare QA testing and patient data protection testing approaches.
Device Diversity: Mobile app testing for healthcare involves testing a Bluetooth connection between a glucose monitor and 500 different types of Android phones through comprehensive integration testing in healthcare.
Real-time apps (like remote heart monitoring) cannot lag, requiring rigorous performance testingof healthcare systems.
Challenge: Simulating unstable networks (e.g., a patient driving through a tunnel) through network testing healthcare scenarios.
Solution: "Network Throttling" tools that artificially degrade the internet connection during healthcare application testing to ensure the app handles the packet loss gracefully without crashing or losing data through reliability testing of medical devices.
Challenge: The "Attack Surface" is huge—from the hospital Wi-Fi to the doctor's iPad to the MRI machine's USB port, requiring comprehensive healthcare cybersecurity testing.
Solution: Continuous Security Testing (DevOps testing healthcare). Security testingin medical is not a final check; it is embedded in every stage of development through CI/CD testing in healthcare. Automated scanners check for code vulnerability testing medical software every time a developer saves their work through penetration testing healthcare applications and encryption testing healthcare apps.
Leading voices in the US emphasize a shift from "Defect Detection" to "Defect Prevention" in healthcare application testing services. Experts argue that healthcare QA testing must be involved in the design phase through software requirements verification testing. If a requirement is "The system shall be secure," a tester asks, "How specifically? 2FA? Biometrics?" through design control verification. This early questioning prevents vague code from being written in the first place.
Senior QA engineers note that the biggest friction point is "Alert Fatigue" in doctor software testing and hospital app testing. Their review of hospital apps often focuses on reducing the noise—ensuring that the software only beeps when it truly matters through clinical workflow testing. They advocate for "Usability Labs" where they watch real doctors try to use the software in mock emergency scenarios to find UI frustrations through usability testing medical software and GUI testing medical devices.
Doctors view software testing healthcare as a form of malpractice insurance. They want to know, "If I follow this app's recommendation and it's wrong, who is liable?" Their feedback pushes testers to ensure that the software provides "Explainable AI"—showing the reasoning behind a diagnosis, not just the answer, so the doctor remains the final authority through clinical decision support testing and SaMD software verification.
A major hospital network in Chicago implemented a new triage app requiring comprehensive hospital app testing. Initial healthcare application testing revealed that nurses were spending too much time clicking through menus. The QA team ran "Time-and-Motion" studies and redesigned the workflow through usability testing of medical software.
Result: The optimized app reduced the time-to-triage significantly. The "45%" improvement referred to the reduction in patient wait times during peak ER hours, directly attributed to the streamlined software validated by healthcare software testing and clinical workflow testing.
A Canadian telehealth provider noticed a spike in pharmacy callbacks due to unclear prescriptions requiring improved doctor software testing.
Action: They implemented rigorous automated testing in a healthcare suite for their e-prescription module, specifically testing for "Sound-Alike Look-Alike" (LASA) drug names through clinical decision support testing.
Result: The software began auto-flagging potential confusion points, drastically reducing the error rate and enhancing pharmacist trust in the digital scripts through effective healthcare application testing and software verification testing.
A multi-specialty clinic in Dubai faced long queues at the front desk, requiring optimization through receptionist app testing.
Action: Performance testing healthcare revealed that the "Insurance Verification" API was timing out during peak hours, freezing the receptionist's screen. The QA team worked with developers to implement asynchronous processing (background checks) through API testing for healthcare integration.
Result: Check-in times dropped, allowing the clinic to handle a higher volume of patients without hiring extra staff, significantly boosting operational efficiency through effective healthcare software testing and integration testing in healthcare.

AI in Testing: "Self-Healing Scripts" through automated testing framework solutions. If a button on the app moves, AI-powered test automation tools in healthcare will recognize it and adjust the test automatically, rather than failing and requiring human repair.
Blockchain: Will be used to test the "Immutable Audit Trails" through healthcare data integrity testing. Software testing healthcare will verify that once a medical record is written to the blockchain, it truly cannot be altered, providing the ultimate data validation testing proof.
Digital Twins: Creating a virtual replica of a human body or a hospital network to run millions of simulations through advanced system testing medical software. We can test how a pacemaker software update affects a "virtual heart" before ever touching a real patient through medical device software testing and SaMD software verification.
The future is "Hospital at Home," requiring new approaches to healthcare application testing. Medical domain testing will move out of the lab and into the living room. We will see a surge in testing for consumer wearables (smartwatches, rings) that feed clinical-grade data to doctors through mobile health app testing. The challenge will be validating the quality of data coming from uncontrolled environments through data validation testing and reliability testing of medical devices.
Standardization: We will likely see a global unification of healthcare regulatory compliance testing standards (like a global FDA) to allow software to be sold across borders more easily through unified device testing services and medical device certification requirements.
Ethical AI Testing: A new role of "AI Ethicist Tester" will emerge to audit medical algorithms for bias and fairness through specialized clinical decision support testing and SaMD software verification.
Quantum Computing: As quantum computers arrive, they will break current encryption. Security testing medical will shift to "Post-Quantum Cryptography" to future-proof patient data protection testing through advanced encryption testing healthcare apps and healthcare API security testing.
Software testing healthcare is the currency of trust in the digital health economy. Without it, the most advanced AI diagnostic tool is just a risky experiment through unvalidated clinical decision support testing. As healthcare globalizes—with US doctors treating patients in rural India via satellite—healthcare application testing ensures that the technology translates safely across borders, languages, and regulations through comprehensive healthcare compliance testing.
It is the invisible infrastructure that allows digital health to scale from a novelty to a necessity through rigorous medical domain testing, healthcare domain testing, and device quality assurance protocols.
In the end, medical device software testing and healthcare application testing are about protecting the most vulnerable aspect of human existence: our health. It is a discipline that demands perfection because the cost of failure is not financial—it is human. By upholding the highest standards of accuracy, safety, and healthcare regulatory compliance testing, testers are not just finding bugs through software verification testing; they are saving lives, one line of code at a time, through comprehensive medical device QA validation.
The future of medicine is digital, but the heart of that future relies entirely on the assurance that the software will work when it matters most through rigorous healthcare QA services, medical testing solutions, device testing services, software validation testing, and comprehensive quality assurance of medical devices. From FDA medical device testing to HIPAA compliance testing, from electronic health records testing to telemedicine software testing USA, every aspect of healthcare software testing contributes to a safer, more effective healthcare ecosystem that benefits patients, providers, and society as a whole.